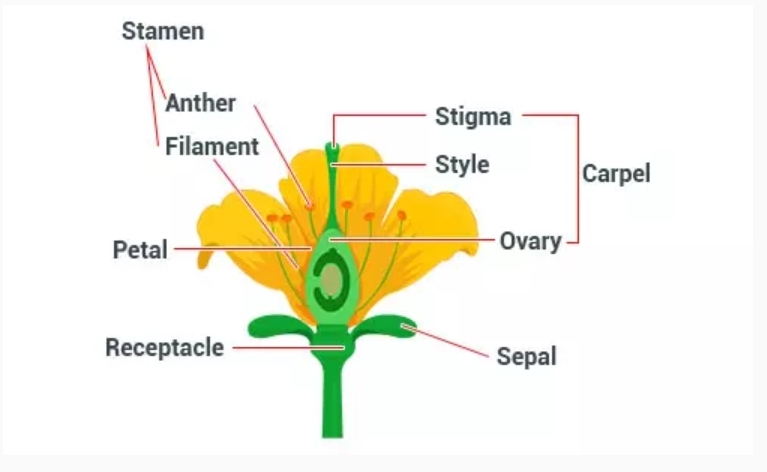
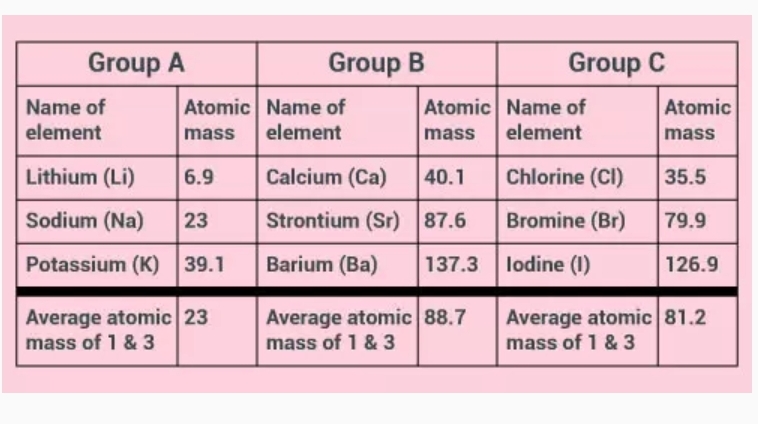
5. Periodic Classification of Elements Dobereiner’s Triads Dobereiner was one of the earliest scientists to find the periodicity of some elements. He arranged elements that have similar properties in groups (or “triads”) of three elements each. In these triads: • The elements were arranged in order of increasing atomic mass. • The average atomic mass of the first and third elements was close to the atomic mass of the second element. • The properties of elements were same. The three groups of Dobereiner’s triads are given in the table at the end of the page. Dobereiner’s triads, however, had several demerits. These are: • Dobereiner could identify only three such triads or groups even though there were several other elements that were known at that time. Some well-known metals like manganese, iron, copper, cobalt, nickel, zinc, silver and gold did not find a place in his triads. • Carbon has atomic mass of 12, nitrogen has 14 and oxygen has 16. According to Dobereiner’s...