Revision Tool Periodic Classification of Elements
5. Periodic Classification of Elements
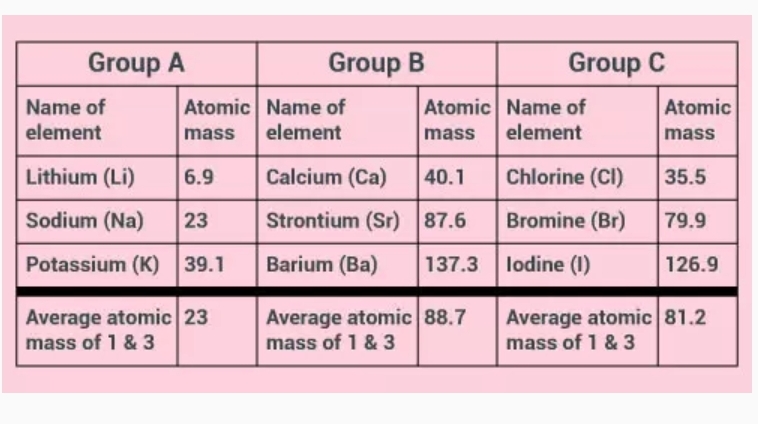
Dobereiner’s Triads
Dobereiner was one of the earliest scientists to find the periodicity of some elements.
He arranged elements that have similar properties in groups (or “triads”) of three elements each. In these triads:
• The elements were arranged in order of increasing atomic mass.
• The average atomic mass of the first and third elements was close to the atomic mass of the second element.
• The properties of elements were same.
The three groups of Dobereiner’s triads are given in the table at the end of the page.
Dobereiner’s triads, however, had several demerits. These are:
• Dobereiner could identify only three such triads or groups even though there were several other elements that were known at that time. Some well-known metals like manganese, iron, copper, cobalt, nickel, zinc, silver and gold did not find a place in his triads.
• Carbon has atomic mass of 12, nitrogen has 14 and oxygen has 16. According to Dobereiner’s rule, these three elements form a triad as well but their properties are completely different.
Because of these reasons, Dobereiner’s triads were discarded.
Trends in Modern Periodic Table
In the modern periodic table, as we move from left to right:
• Atomic number and atomic mass of the elements increases, while atomic radius decreases
• Metallic character decreases while non-metallic property and electronegativity of the elements increases
Newland’s Octaves
Newland’s Octaves
In 1865 John Newland arranged all the elements known at the time in order of increasing atomic mass. He found that every eighth element in the series exhibited similar properties. Newland compared them with the octaves of the piano and so the table was called Newland’s Octaves.
Newland’s octaves had several limitations, including:
• The periodicity of elements was valid only till calcium. After calcium, every 8th element did not exhibit the same properties.
• At the time, there were 56 known 56 elements and Newland assumed no more elements would be discovered, which was subsequently proven incorrect.
• Some element like cobalt and nickel were placed together in the same slot although their properties were very different.
These limitations made Newland’s Octaves an ineffective method of arranging the elements.
Moseley’s Modern Periodic Table
Moseley’s Modern Periodic Table
Mendeleev’s periodic table arranged the elements based on their atomic mass, which is the sum of the number of protons and neutrons present in the nucleus. After failure of Mendeleev’s table, Henry Moseley made an attempt to organise the elements on the basis of their atomic number alone, which is the number of protons present in an element’s nucleus (ignoring neutrons). This arrangement eliminated a number of the shortcomings in Mendeleev’s table.
Moseley arranged the elements in a table form which is given in the image at the end of the page.
In the modern periodic table, the seven rows are called the periods and the eighteen columns are called groups. The number of elements accommodated in each period is given in the table A.
All the elements within a group have the same number of valence electrons and hence valency.
Table B shows the electronic configuration of the first 20 elements in the periodic table.
Mendeleev’s Periodic Table
Mendeleev’s Periodic Table
Dmitri Mendeleev arranged the elements on the basis of their atomic masses. He also conducted chemical reactions of each of these elements with hydrogen and oxygen. Then, taking atomic mass as the base and using chemical properties such as for mation of hydrides and oxides, Mendeleev arranged all the then known elements in a table which had eight columns, or groups, and six rows, or periods.
Some of the key achievements of Mendeleev’s periodic table include:
• Mendeleev’s periodic table had blank spaces for elements that were not known at the time. This gave scope for placing more elements as and when they were discovered. He added the prefix eka to the name of the element preceding the blank space. For example, there is a blank space after silicon, which he called eka-silicon.
• He made predictions about the properties of the missing elements such as:
· Atomic mass
· Density
· Melting point
· Oxide’s formula
· Chloride’s formula
His predictions regarding those missing elements were very accurate.
• Noble gases were discovered much later, but they could be placed in a separate group on the basis of their reactivity without disturbing the Mendeleev’s order.
• On the basis of chemical properties, cobalt with a higher atomic mass was placed before nickel with lower atomic mass.
However, Mendeleev’s periodic table had some demerits as well:
However, Mendeleev’s periodic table had some demerits as well:
• In Mendeleev’s periodic table, hydrogen is placed with alkali metals because hydrogen reacts with halogens just like alkali metals do. However, hydrogen also reacts with alkali metals the way halogens do, so it could just as easily have been classified with halogens. Hence the placement of hydrogen with alkali metals was not necessarily correct.
• Isotopes of an element have different atomic masses but same atomic number and chemical properties. As Mendeleev’s periodic table is based on atomic masses of the elements, the placement of isotopes of an element was not clear.
• In some cases, Mendeleev placed elements according to their properties, but these did not follow the order of increasing atomic mass.
Although it was very accurate in many cases, because of these limitations, Mendeleev’s periodic table was not adopted as a method of arranging elements.
Categorisation of Elements in Modern Periodic Table
Categorisation of Elements in Modern Periodic Table
• All the elements which have 1, 2 or 3 electrons in their outermost shell are metals except hydrogen and helium. Metals have a tendency to lose 1, 2 or 3 electrons from their outermost shells to become positively charged ions or cations. Example: sodium. These are found in the left, centre or lower parts of the table.
• The elements which have 4, 5, 6 or 7 electrons in their outermost shell are non-metals. Non-metals have a tendency to gain 1, 2 or 3 electrons into their outermost shells to become negatively charged ions or anions. Example: fluorine. Non-metals are present in the top and right side of the periodic table.
• Some elements which display the properties of both metals and non-metals are called metalloids such as boron, silicon and germanium. In the case of these metalloids, the electronic configuration alone does not determine whether they are metals or non-metals.
• The elements of group 1, which have 1 electron in their outermost shell, are alkali metals (except hydrogen which is a non-metal). Alkali metals are highly reactive as they can easily complete their octet by losing 1 electron and forming a bond with other ions.
• The elements of group 17, which have 7 electrons in their outermost shell, are halogens. Halogens are highly reactive non-metals as they can easily complete their octet by gaining one electron and forming bonds with other ions. Because of their reactivity, halogens generally exist as compounds, rather than as pure elements.
• The elements with 8 electrons in their outermost shell are noble gases (also helium which has two electrons). These are stable elements and usually do not react chemically with other elements.
• The atoms of hydrogen and helium in which the valence shell K has two electrons are said to have completed their duplet. Atoms of all other elements have the capacity to accommodate eight electrons in their valence shell and are said to have completed their octet.





Comments
Post a Comment